Table of Contents
ToggleMaking Organic Matter in Plants
Just like other living things, plants also have to provide energy in order to maintain their vitality. This required energy is provided by the use and burning of organic foods. Continuously finding these organic compounds to be used in energy supply is essential for plant nutrition and continuation of life. In general, plants make these foods themselves, thanks to their special chemical abilities.
Making the organic substances necessary for themselves from the inorganic substances they take from the external environment is called assimilation. Living things are called autotrophic if they can show the ability to do this on their own. All green plants have this ability. Organisms that cannot make their own food are called heterotrophs.
Living things that are autotrophs make the organic substances necessary for themselves by using the carbon dioxide they take from the air by making use of a certain energy. This very important event is called carbon dioxide assimilation or, in other words, carbon dioxide assimilation. If the energy required for this work is obtained from the sun, this event is called photosynthesis.
Photosynthesis
The main source of chemical energy stored in organic matter is the sun. The physical energy of the sun is converted into chemical bond energy in organic matter. This work is performed only by living things that carry chlorophyll. These creatures use the sun as an energy source and convert carbon dioxide into organic matter. Thus, it converts solar energy into chemical bond energy. This phenomenon is called photosynthesis.
The continuation of the life of living things on earth consists of energy changes in one aspect. Plants maintain their vitality by breaking down organic nutrients and converting the chemical energy (ATP) in them into different energies such as heat, motion, etc.
One of the most important events occurring in nature is photosynthesis. Under the influence of sunlight, green plants combine carbon dioxide and water to form carbohydrates as the end product of their metabolism. The resulting carbohydrates are used as a free energy source in plants. Photosynthesis can be expressed with a simple chemical equation as follows.
As can be understood from this general equation of photosynthesis, chlorophyll is necessary for sunlight retention. In other words, cells without chlorophyll cannot perform photosynthesis. Parts of plants such as roots and woody stems cannot photosynthesize. The most photosynthesizing parts are leaves. Photosynthesis not only ensures the production of organic substances, but also maintains a constant rate of atmospheric gases. The harmful gas carbon dioxide in the air is taken and replaced with oxygen, which most of the living things need.
Chlorophyll
Chlorophylls are the most active colorants involved in photosynthesis. Chlorophylls are found in all parts of plants that appear green, but are mostly found in leaves. Therefore, the organs specialized for photosynthesis are leaves.
Chlorophylls are found in the chloroplasts of the plant cell. Chlorophyll has the ability to absorb light energy and convert it to chemical energy and store it. There are about twenty types of chlorophyll and the most active ones in plants are “a and b” chlorophylls.
Chlorophylls contain C (carbon), H (hydrogen), O (oxygen), N (nitrogen) and Mg (magnesium). The difference between chlorophylls is due to the oxygen and hydrogen numbers. Mg and N are required for the synthesis of chlorophyll. In addition, although it is not used in its structure, Fe (iron) is used in intermediate products. Chlorophyll-a operates in high light. Chlorophyll-b operates in low light.
In autumn, the amount of enzymes that break down chlorophyll in plant cells increases. Chlorophylls break down and leaves turn yellow.
Phases of Photosynthesis
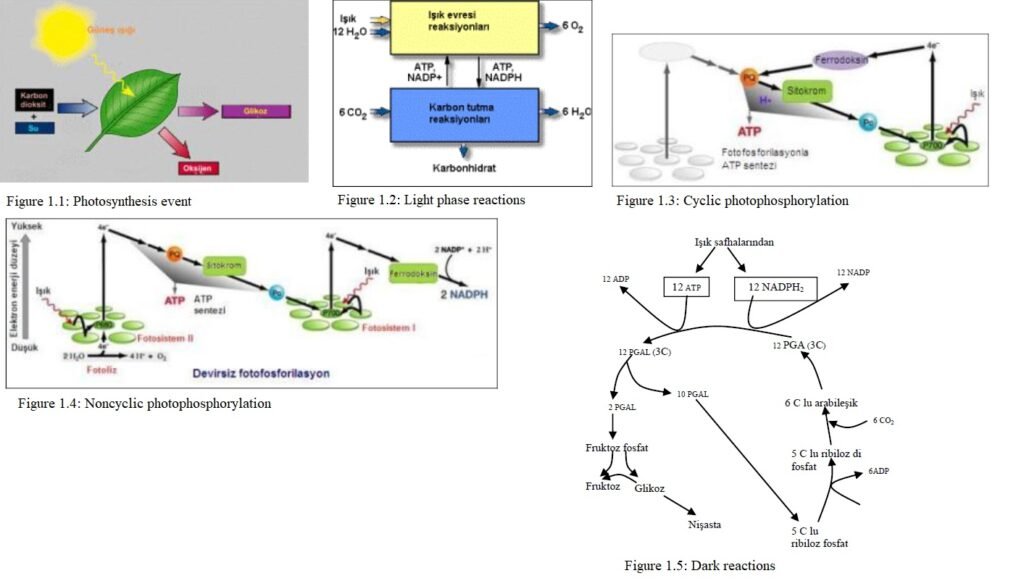
Photosynthesis involves a large number of successive chemical reactions. Photosynthesis reactions are divided into two parts, light and dark reactions. Light reactions require water and light. These reactions do not occur without light. In dark reactions, carbon dioxide molecule is needed. These reactions are called the “dark cycle” because no light is used. Most dark-circuit reactions occur in light environments, along with light reactions.
Light Reactions
In this phase, reactions take place with the effect of light energy. Enzymes are not used. This phase does not occur without light. The reaction begins when the chlorophylls in the part of the chloroplast called the grana absorb the sun’s energy. The purpose of light reactions is to create ATP (energy) and NADPH2. The synthesis of ATP using light energy is called photophosphorylation. There are two types of photophosphorylation.
Cyclic photophosphorylation
Electrons are emitted from the chlorophyll molecule that is excited by light. The electron is captured by ferredoxin, a coenzyme, and transferred to the cytochrome system. Electrons are converted from cytochromes to chlorophyll. Thus, chlorophyll gains the electron it lost and the cycle is completed. ATP is synthesized from the energy released during the oxidation and reduction reactions that take place in electron transfer. Electrons leaving chlorophyll are called cyclic photophosphorylation because they are returned to chlorophyll.
ADP+P ATP+H2O
Noncyclic photophosphorylation
The electron that is detached from the chlorophyll does not return again. The lost electron is obtained from another source. Therefore, this phase is called acyclic photophosphorylation.
Two pigment systems are involved in noncyclic photophosphorylation. These pigment systems are PS-1 and PS-2. PS-1 is in chlorophyll-a, PS-2 is in chlorophyll-b.
When PS-1 is excited by light, the ejected electron is transferred to ferredoxine. Ferredoxin takes the electron from NADP (nicotine amide adeine dinucleotide phosphate) to form NADPH2. The electron lost by PS-1 does not come back. PS-1 takes the lost electron from PS-2.
When PS-2 is excited by light, the separated electron is taken up by the plastoquinone. Plastoquinone transfers this electron to cytochromes. Cytochromes donate electron PS-1. It gains the electron it lost in PS-1. When the cytochromes donate the electron to PS-1, one mole of ATP is synthesized.
When PS-2 donates its electron, it becomes an electron acceptor. PS-2 completes its missing electron from hydroxyl ions formed by the ionization of water. Hydroxyls that donate their electrons combine to form water and oxygen.
2 NADP + 2 ADP + 2P + 2H20 2NADPH2+ 2 ATP + 02
Dark Reactions
Dark reactions are enzymatic reactions. It takes place in the part of the chloroplast called the stroma. There is no need for light in this phase. In this phase, CO2, ATP and NADPH2 synthesized in the light phase are used. In each reaction, different enzymes come into play.
At the beginning of the dark reactions, ribulosephosphate (5C) in the environment is activated by the addition of P separated from an ATP molecule. Ribulosediphosphate combines with CO2 from the air. An unstable six-carbon intermediate is formed. This compound is split into two by enzymes. Three-carbon phosphoglyceric acid (PGA) is formed. Phosphoglyceric acid is converted to phosphoglyceraldehyde (PGAL) by enzymes. During this transformation, ATP and NADPH2s synthesized in the light phase are used.
Fructose is formed from a part of the PGAL and ribulose phosphate from a part. As ribulosephosphates undergo new reactions, fructose is converted to glucose. Starch is also formed from glucose. When necessary in plants, it creates amino acids, pyruvic acid and fatty acids from PGAL.
Chemosynthesis
The production of organic matter without the use of solar energy is called chemosynthesis. Some non-green primitive plants cannot obtain the necessary energy from the sun to make the organic nutrients they need (to use the carbon dioxide they get from the air). Although rare, such plants provide the energy required in this event, chemically and by oxidizing some elements from their environment. These primitive plants spend the energy they obtain by oxidizing chemical elements in this way in the use of carbon dioxide. In this way, they synthesize organic compounds from inorganic compounds.
Nitrogen is a very important element for plants. Plants can obtain nitrogen only from nitrite and nitrate salts in the soil. Nitrogen in the soil, on the other hand, is formed from the decay of plant and animal organic wastes and is in the form of ammonia. Plants cannot benefit from this nitrogen. For nitrogen to be usable, ammonia must become nitrite and nitrate salts. This is done by nitrite and nitrate bacteria living in the soil. First, nitrite bacteria oxidize ammonia to nitrite acid.
2NH3 +CO2 NO2 (Nitrite) + H2O + 158 cal. (Energy Recovery)
The energy released from this reaction is used to combine water and carbon dioxide. Thus, living things make the organic nutrients they need. H20 + CO2 + K.cal. Glucose + 02 (Nutritional Synthesis)
Factors Affecting the Rate of Photosynthesis
We can divide the factors affecting the rate of photosynthesis into two groups.
Environmental factors
We can count it as temperature, water (more in sweating), carbon dioxide density, light intensity and wavelength of light. Temperature is effective at high light intensity. The ideal temperature for photosynthesis is approximately 10-40°C.
Increasing the carbon dioxide concentration increases the rate of photosynthesis. After a certain level, the rate of photosynthesis remains constant even if carbon dioxide increases.
As the light intensity increases, the rate of photosynthesis also increases, but after a certain limit, the rate of photosynthesis remains constant even if the light intensity increases. The rate of photosynthesis is maximum in blue, violet and red light. Photosynthesis does not occur in infrared and ultraviolet rays.
The amount of CO2 also increases the rate of photosynthesis up to a certain amount.
Genetic factors
Factors such as the amount of chlorophyll, the structure of the stomata, enzymatic factors, and the thickness of the cuticle layer are genetic factors.